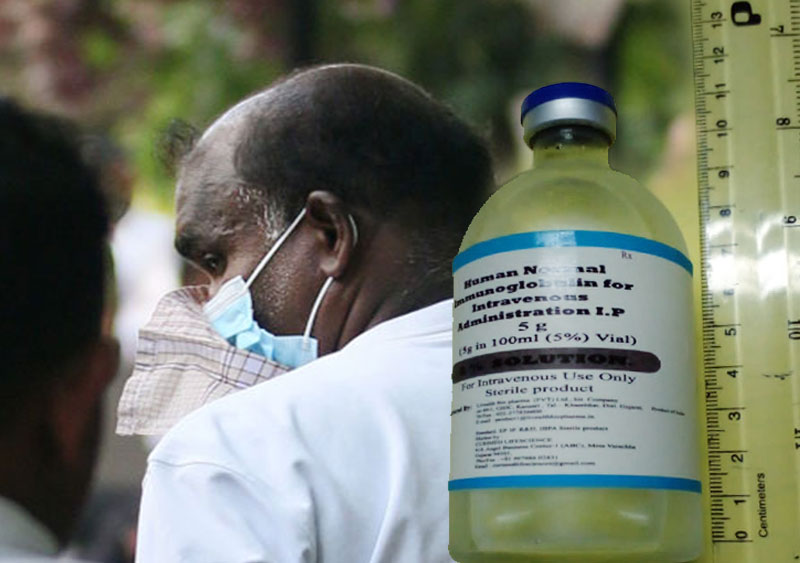
The owner of the company, which allegedly used forged documents to import a batch of substandard human intravenous immunoglobulin (IVIG), has been remanded until November 15.
The accused known as Aruna Deepthi, who was arrested by the Criminal Investigations Department (CID), was produced before Maligakanda Magistrate’s Court yesterday (01).
On Monday, the court imposed overseas travel bans on the owner of the company, which is accused of using forged documents to import a batch of vials of substandard immunoglobulin, and two other high-ranking government officials who were allegedly involved in this fraudulent activity.
Accordingly, Sugath Janaka Fernando, also known as ‘Aruna Deepthi’, Chief Executive Officer of the National Medicines Regulatory Authority (NMRA) Dr. Vijith Gunasekara and Director (Supplies Division) of the Ministry of Health Dr. Kapila Wickramanayake were barred from flying out of the country.
Earlier this month, the National Medicines Regulatory Authority (NMRA) said that forged documents were found to have been submitted for Customs clearance when importing the drug which later failed the quality tests.
The product, which was said to have been manufactured by Livealth Biopharma Pvt Ltd. India, was imported by a local medicine supplier called Isolez Biotech Pharma AG (Pvt) Ltd.
However, the India-based manufacturer has denied having to do anything with this fraudulent activity and has communicated to the NMRA that it has neither manufactured, supplied nor exported these products to any party.
It was found that funds amounting to Rs. 130 million were misappropriated through the unlawful importation of 22,500 vials of IVIG.
The local medicines regulator recently said the situation came to light following reports of allergic reactions after the drug was administered to several patients being treated at the Colombo National Hospital and the Matale District Hospital on August 22 and September 16, respectively.
New directives on medicines purchases
Meanwhile, Minister of Health Ramesh Pathirane has issued new directives to Health Sector officials on the purchase of medicines.
Instructions have been issued to bring only registered medicines into Sri Lanka and in the event of no registered medicines, to check the necessary data on the said drugs and purchase them with the approval of the National Medicines Regulatory Authority (NMRA).
The directive was issued to officials from the NMRA, State Pharmaceuticals Corporation, State Pharmaceutical Manufacturing Corporation, and Institutes of Medical Supply Division during a discussion with senior officials of the Ministry of Health yesterday.
Health Minister Ramesh Pathirane warned that if someone acts outside of the above system, necessary measures will be taken against them.
Officials were informed to suspend the purchase of drugs under emergency purchases and to arrange for the delivery of drugs that are in short supply as soon as possible through the prescribed method.
The Minister of Health further informed the relevant officials to pay attention to the essential drugs used for cancer, kidney diseases, etc., to maintain these drugs without shortage, and to constantly pay attention to all necessary drugs.
(With inputs from Adaderana and Newswire)